- The stem cells were created from cells of young mice
- Researchers put these blood cells in an acidic environment
- Stem cell therapies may one day be used to treat diseases
- This new technique has not been tried in humans
(CNN) -- We run too hard, we fall down, we're sick -- all of this puts stress on the cells in our bodies. But in what's being called a breakthrough in regenerative medicine, researchers have found a way to make stem cells by purposely putting mature cells under stress.
Two new studies published Wednesday in the journal Nature describe a method of taking mature cells from mice and turning them into embryonic-like stem cells, which can be coaxed into becoming any other kind of cell possible. One method effectively boils down to this: Put the cells in an acidic environment.
"I think the process we've described mimics Mother Nature," said Dr. Charles Vacanti, director of the laboratory for Tissue Engineering and Regenerative Medicine at Brigham & Women's Hospital in Boston and senior author on one of the studies. "It's a natural process that cells normally respond to."
Both studies represent a new step in the thriving science of stem cell research, which seeks to develop therapies to repair bodily damage and cure disease by being able to insert cells that can grow into whatever tissues or organs are needed. If you take an organ that's functioning at 10% of normal and bring it up to 25% functionality, that could greatly reduce the likelihood of fatality in that particular disease, Vacanti said.
This method by Vacanti and his colleagues "is truly the simplest, cheapest, fastest method ever achieved for reprogramming [cells]," said Jeff Karp, associate professor of medicine at the Brigham & Women's Hospital and principal faculty member at the Harvard Stem Cell Institute. He was not involved in the study.
Scientists grow minibrains from stem cells
Before the technique described in Nature, the leading candidates for creating stem cells artificially were those derived from embryos and stem cells from adult cells that require the insertion of DNA to become reprogrammable.
Stem cells are created the natural way every time an egg that is fertilized begins to divide. During the first four to five days of cell division, so-called pluripotent stem cells develop. They have the ability to turn into any cell in the body. Removing stem cells from the embryo destroys it, which is why this type of research is controversial.
Researchers have also developed a method of producing embryonic-like stem cells by taking a skin cell from a patient, for example, and adding a few bits of foreign DNA to reprogram the skin cell to become like an embryo and produce pluripotent cells, too. However, these cells are usually used for research because researchers do not want to give patients cells with extra DNA.
The new method does not involve the destruction of embryos or inserting new genetic material into cells, Vacanti said. It also avoids the problem of rejection: The body may reject stem cells that came from other people, but this method uses an individual's own mature cells.
"It was really surprising to see that such a remarkable transformation could be triggered simply by stimuli from outside of the cell," said Haruko Obokata of the Riken Center for Developmental Biology in Japan in a news conference this week.
The process is called STAP, which stands for "stimulus-triggered acquisition of pluripotency." Karp estimates that the method is five to 10 times faster than other means of reprogramming cells.
Have a taste of the world's first stem cell burger
Researchers used mice to study the STAP cell phenomenon. They genetically altered the mice donating stem cells to "label" those cells with the color green. For instance, they modified mice such that their cells would light up green in response to a particular wavelength of light.
The scientists exposed blood cells from these genetically altered mice to an acidic environment. A few days later, they saw that these cells turned into the embryonic-like state and grew in spherical clusters.
Scientists put the cell clusters into a mouse embryo that had not been genetically modified. It turned out, the implanted clusters could form tissues in all of the organs that the researchers tested. The scientists knew that the cells came from the original mouse because they turned green when exposed to a particular light.
Besides modifying acidity, researchers also stressed the cells in other ways, such as lowering the oxygen environment and disrupting the cell membrane. Increasing acidity was one of the most effective methods of turning mouse blood cells into STAP cells.
There are, of course, some caveats.
For now, the STAP cell procedure has only been demonstrated in cells from young mice. The effectiveness in humans, and the risks, are unknown.
Researchers have not yet shown how STAP embryonic-like stem cells compare with bona fide embryonic stem cells or induced pluripotent stem cells, Karp said.
Also, although the study was "rigorous" and "well-controlled," it did not demonstrate exactly why the stress on the cells caused them to become STAP cells, Karp said.
As with everything in science, more research is required to confirm the findings and learn more about the implications.
Vacanti hopes the process could get tested clinically in humans within three years. He noted that induced pluripotent stem cells are already being explored in Japan in humans and the same "platforms" could be utilized for STAP cells.
STAP cells also have an additional property that embryonic stem cells and induced pluripotent stem cells do not: They can become placental cells. Scientists can manipulate them to contribute to tissues of either the embryo or the placenta.
What therapeutic purpose growing more placenta could serve, Vacanti isn't sure -- unless, that is, you wanted to create an embryo and bring it to term.
Cloning stem cells: What does it mean?
But that's not the goal of this research. Vacanti and colleagues want to explore possible ties to cancer from the STAP cell process; it could potentially help to model the process by which cells become cancerous and explore if there is a way to reverse the process.
Stem cell research as a field has been growing at "lightning speed," Karp said.
New reprogramming approaches to stem cells are emerging all the time, he said, and this one in particular "looks incredibly promising."
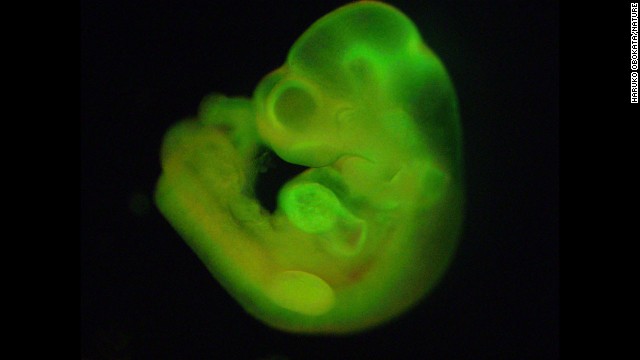 Researchers have developed a new method of making stem cells. Mouse cells were "stressed" in several ways, such as by being placed in an acidic environment. Researchers were then able to use those cells to generate various tissues in developing mice. This image shows a mouse fetus that has tissues that grew, in part, from the stem cells. Click through the gallery to learn more about stem cell research.
Researchers have developed a new method of making stem cells. Mouse cells were "stressed" in several ways, such as by being placed in an acidic environment. Researchers were then able to use those cells to generate various tissues in developing mice. This image shows a mouse fetus that has tissues that grew, in part, from the stem cells. Click through the gallery to learn more about stem cell research. 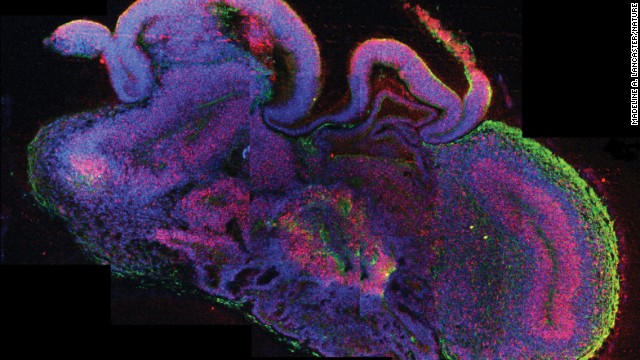 Scientists have created what they are calling "cerebral organoids" using stem cells. These pea-sized structures are made of human brain tissue, and they can help researchers explore important questions about brain development and disorders that occur during these first stages of life.
Scientists have created what they are calling "cerebral organoids" using stem cells. These pea-sized structures are made of human brain tissue, and they can help researchers explore important questions about brain development and disorders that occur during these first stages of life.  Stem cells have the potential to become many different kinds of cells, and can renew themselves through cell division. Scientists view stem cells as a possible gateway to curing many medical conditions, from Parkinson's disease to diabetes. Stem cells are viewed on computer here at UConn Health Center in 2010.
Stem cells have the potential to become many different kinds of cells, and can renew themselves through cell division. Scientists view stem cells as a possible gateway to curing many medical conditions, from Parkinson's disease to diabetes. Stem cells are viewed on computer here at UConn Health Center in 2010. 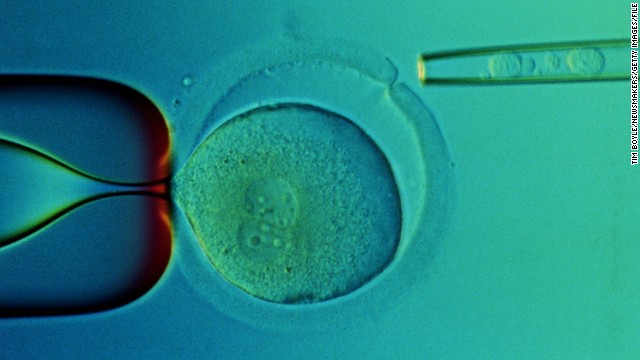 A closeup of a microscope slide taken in 2000 at the Reproductive Genetics Institute's Chicago laboratory shows transplanted stem cells taken from the umbilical cord blood of a baby named Adam Nash. Adam's sister Molly has a genetic disease called Franconi Anemia. Their parents wanted to have a child who could be a stem cell donor for Molly. Using in vitro fertilization, doctors created embryos and then tested them for the genetic disease. They chose one that did not have the disorder, which grew into baby Adam. Molly received a stem cell transplant from stem cells from Adam's umbilical cord. Both children are alive today.
A closeup of a microscope slide taken in 2000 at the Reproductive Genetics Institute's Chicago laboratory shows transplanted stem cells taken from the umbilical cord blood of a baby named Adam Nash. Adam's sister Molly has a genetic disease called Franconi Anemia. Their parents wanted to have a child who could be a stem cell donor for Molly. Using in vitro fertilization, doctors created embryos and then tested them for the genetic disease. They chose one that did not have the disorder, which grew into baby Adam. Molly received a stem cell transplant from stem cells from Adam's umbilical cord. Both children are alive today.  In 1998, then-President Bill Clinton requested a National Bioethics Advisory Commission to study the question of stem cell research.
In 1998, then-President Bill Clinton requested a National Bioethics Advisory Commission to study the question of stem cell research. 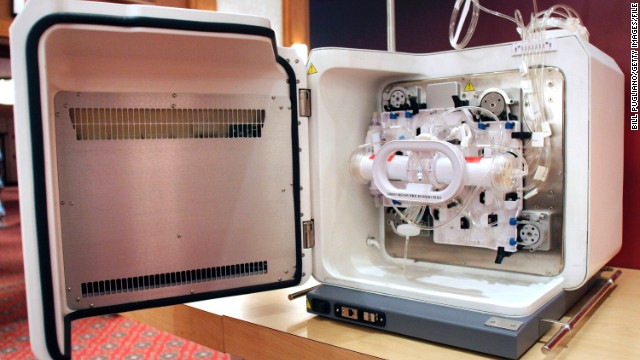 In 2000, The National Institutes of Health issued guidelines for the use of embryonic stem cells in research, specifying that scientists receiving federal funds could use only extra embryos that would otherwise be discarded. President Clinton approved federal funding for stem cell research but Congress did not fund it. Above, a Cell Expansion System which is used to grow cells is seen during the 2010 World Stem Cell Summit in Detroit.
In 2000, The National Institutes of Health issued guidelines for the use of embryonic stem cells in research, specifying that scientists receiving federal funds could use only extra embryos that would otherwise be discarded. President Clinton approved federal funding for stem cell research but Congress did not fund it. Above, a Cell Expansion System which is used to grow cells is seen during the 2010 World Stem Cell Summit in Detroit. 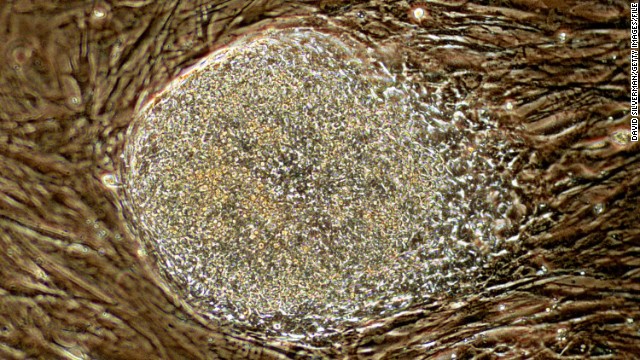 In August 2001, then-President George W. Bush announced he would allow federal funding for about 60 existing stem cell lines created before this date. Above, a human stem cell colony, which is no more than 1mm wide and comprises thousands of individual stem cells, grows on mouse embryonic fibroblast in a research laboratory in September 2001.
In August 2001, then-President George W. Bush announced he would allow federal funding for about 60 existing stem cell lines created before this date. Above, a human stem cell colony, which is no more than 1mm wide and comprises thousands of individual stem cells, grows on mouse embryonic fibroblast in a research laboratory in September 2001. 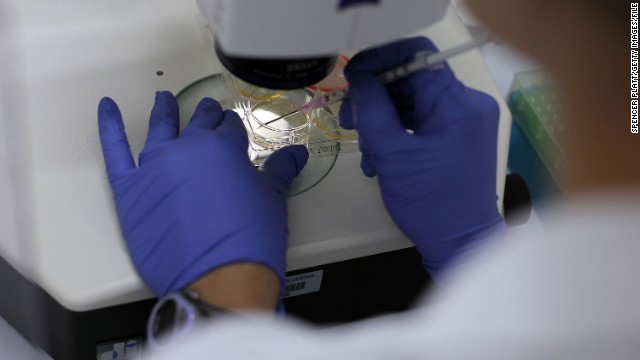 In 2005, Connecticut and Illinois designated state funds to support stem cell research in their states. Above, a woman works on stem cells at the University of Connecticut's Stem Cell Institute at the UConn Health Center in August 2010 in Farmington, Connecticut.
In 2005, Connecticut and Illinois designated state funds to support stem cell research in their states. Above, a woman works on stem cells at the University of Connecticut's Stem Cell Institute at the UConn Health Center in August 2010 in Farmington, Connecticut.  In March 2009, President Barack Obama signed an executive order that removed restrictions on embryonic stem cell research. His action overturned an order approved by President George W. Bush in August 2001 that barred the National Institutes of Health from funding research on embryonic stem cells beyond using 60 cell lines that existed at that time. Above, Obama signs the order.
In March 2009, President Barack Obama signed an executive order that removed restrictions on embryonic stem cell research. His action overturned an order approved by President George W. Bush in August 2001 that barred the National Institutes of Health from funding research on embryonic stem cells beyond using 60 cell lines that existed at that time. Above, Obama signs the order.  In November 2010, William Caldwell, CEO of Advanced Cell Technology, told CNN that the FDA had granted approval for his company to start a clinical trial using cells grown from human embryonic stem cells. The treatment would be for an inherited degenerative eye disease. Above, dozens of packages containing frozen embryonic stem cells remain in liquid nitrogen in a laboratory, at the University of Sao Paulo's human genome research center, in Sao Paulo, Brazil, in March 2008.
In November 2010, William Caldwell, CEO of Advanced Cell Technology, told CNN that the FDA had granted approval for his company to start a clinical trial using cells grown from human embryonic stem cells. The treatment would be for an inherited degenerative eye disease. Above, dozens of packages containing frozen embryonic stem cells remain in liquid nitrogen in a laboratory, at the University of Sao Paulo's human genome research center, in Sao Paulo, Brazil, in March 2008.  In May 2011, stem cell therapy in sports medicine was spotlighted after New York Yankee pitcher Bartolo Colon was revealed to have had fat and bone marrow stem cells injected into his injured elbow and shoulder while in the Dominican Republic. Above, Colon pitches against the Boston Red Sox on in May 2011.
In May 2011, stem cell therapy in sports medicine was spotlighted after New York Yankee pitcher Bartolo Colon was revealed to have had fat and bone marrow stem cells injected into his injured elbow and shoulder while in the Dominican Republic. Above, Colon pitches against the Boston Red Sox on in May 2011. 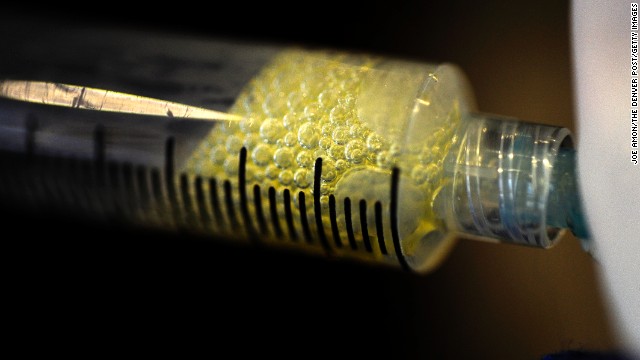 In February 2012, early research published by scientists at Cedars-Sinai Medical Center and Johns Hopkins University showed that a patient's own stem cells can be used to regenerate heart tissue and help undo damage caused by a heart attack. It is the first instance of therapeutic regeneration. Above, fluid is removed from the knee of a patient to collect adult stem cells by at a clinic in Broomfield, Colorado.
In February 2012, early research published by scientists at Cedars-Sinai Medical Center and Johns Hopkins University showed that a patient's own stem cells can be used to regenerate heart tissue and help undo damage caused by a heart attack. It is the first instance of therapeutic regeneration. Above, fluid is removed from the knee of a patient to collect adult stem cells by at a clinic in Broomfield, Colorado.  In October 2012, Sir John Gurdon and Shinya Yamanaka were awarded the Nobel Prize for Physiology or Medicine for discovering how to make pluripotent stem cells. They both showed that cells could be reprogrammed after they had already specialized. This changed scientists' understanding of how cells and organisms develop. Above, Sir Gurdon speaks at a press conference after being awarded the Prize.
In October 2012, Sir John Gurdon and Shinya Yamanaka were awarded the Nobel Prize for Physiology or Medicine for discovering how to make pluripotent stem cells. They both showed that cells could be reprogrammed after they had already specialized. This changed scientists' understanding of how cells and organisms develop. Above, Sir Gurdon speaks at a press conference after being awarded the Prize. 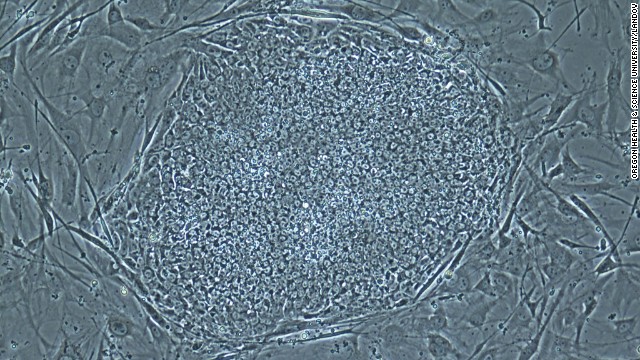 On May 16, 2013, scientists announced that they had, for the first time, produced embryos using skin cells, and then used the embryos to make stem cell lines. This technique resembles what was used in cloning Dolly the sheep, but the earlier technique could not have led to a fully-cloned human baby. Above, a photo provided by the Oregon Health & Science University shows a stem cell colony produced from human skin cells.
On May 16, 2013, scientists announced that they had, for the first time, produced embryos using skin cells, and then used the embryos to make stem cell lines. This technique resembles what was used in cloning Dolly the sheep, but the earlier technique could not have led to a fully-cloned human baby. Above, a photo provided by the Oregon Health & Science University shows a stem cell colony produced from human skin cells.  On Tuesday, August 5, the world's first stem cell burger was cooked and eaten in London. The brainchild of Maastricht University's Mark Post, the burger was made of 20,000 small strands of meat grown from a cow's muscle cells and took three months to create and cost $330,000 to develop.
On Tuesday, August 5, the world's first stem cell burger was cooked and eaten in London. The brainchild of Maastricht University's Mark Post, the burger was made of 20,000 small strands of meat grown from a cow's muscle cells and took three months to create and cost $330,000 to develop. 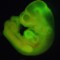










No comments:
Post a Comment